According to the Clevelad Clinic, A ventricular septal defect (VSD) is a hole in the wall (septum) that separates the heart’s two lower chambers (ventricles). Ventricular septal defects usually occur by themselves, without other birth defects of any kind. Experts estimate that VSDs account for about 30 percent of all congenital heart defects, occurring in 1 out of every 500 babies.
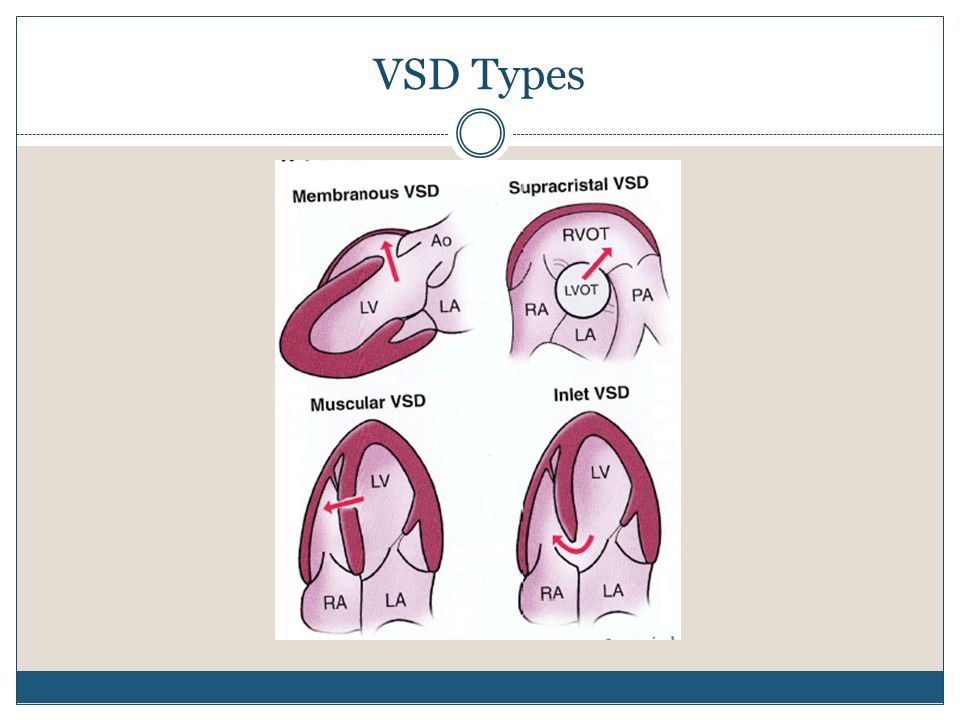
There are four basic types of VSD:
- Membranous VSD. An opening in a particular area of the upper section of the ventricular septum (an area called the membranous septum), near the valves. …
- Muscular VSD. …
- Atrioventricular canal type VSD. …
- Conal septal VSD.
A perimembranous has the membranous septum incorporated into its postero-inferior border.
Muscular VSDs are completely surrounded by muscle and can be located anywhere in the muscular ventricular septum.
Subarterial VSDs(synonyms: supracristal, doubly committed or juxta arterial defects) are immediately adjacent to both the aortic and pulmonary valves and may have perimembranous or muscular border.
Patients with certain genetic abnormalities, e.g., Down syndrome, have a high incidence of associated VSDs: VSD is an integral part of tetralogy of Fallot, and VSDs are present in most patients with univentricular circulation or transposition of the great arteries.
VSDs with a maximal size of 2 mm or less are likely to close prenatally, while others may close within the first 10 years of life [21]. Residual scarring in the septum or scarring with aneurysmal dilatation of the membranous septum with redundant tricuspid valve tissue may be seen at autopsy at the site of spontaneously closed defect.
Persistent VSDs are often restrictive but may be larger. Large or multiple VSDs can lead to pulmonary hypertension and right ventricular hypertrophy, eventually with the development of Eisenmenger syndrome characterized by shunt reversal and cyanosis. Patients with a VSD and significant, irreversible pulmonary vascular disease are deemed inoperable.
For this reason, timely surgical closure is recommended for all large perimembranous VSDs, supracristal VSDs, and VSDs with aortic valve prolapse [22]. Muscular VSDs may be closed by percutaneous techniques. A large number of devices have been used for VSD occlusion, the Amplatzer VSD occlude device being the most popular (Table 1). https://link.springer.com/article/10.1007/s00428-020-02779-8/figures/1
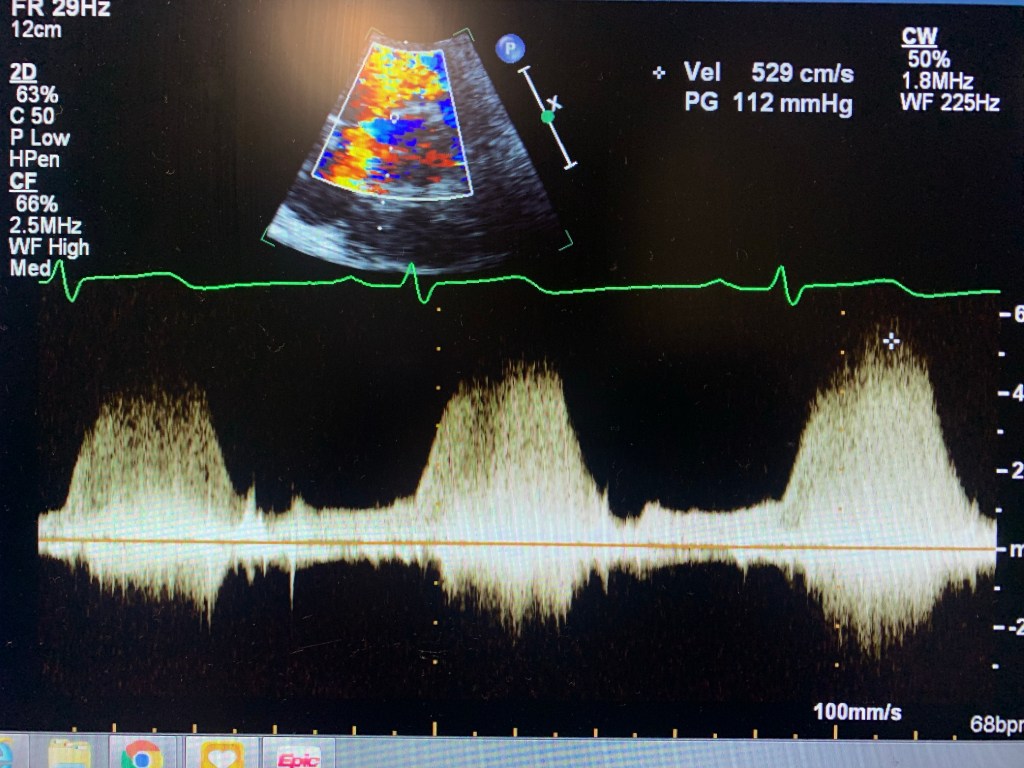
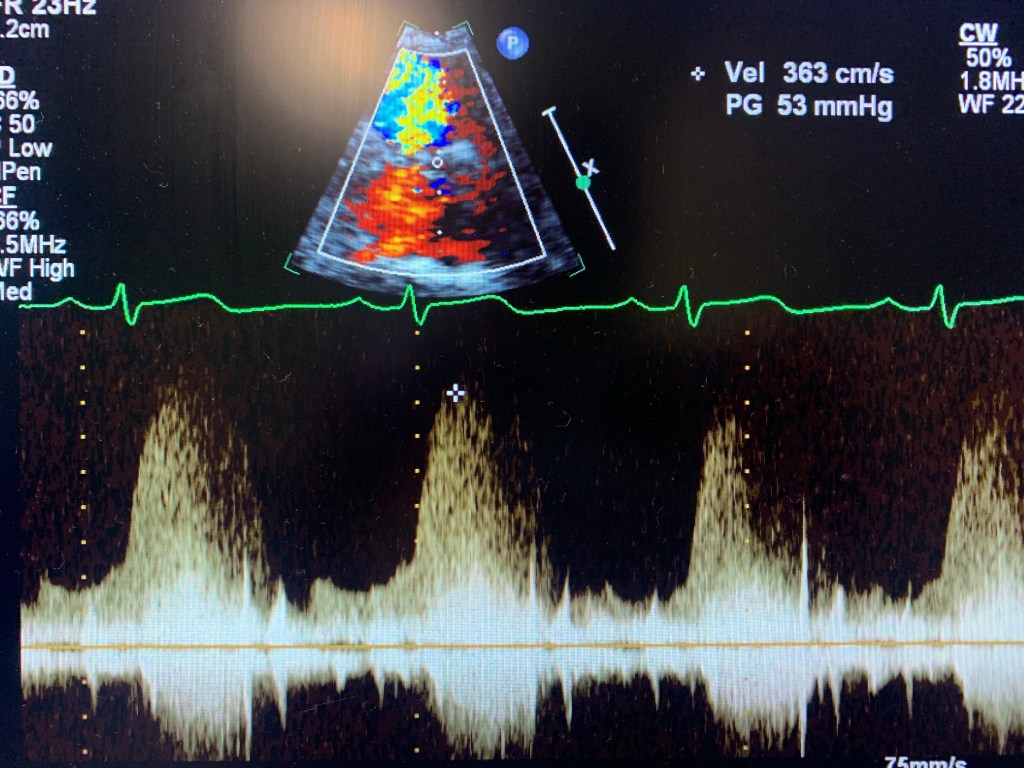
Echocardiogram of a VSD
Case study: a 32 y/o female, w/ shortness of breath, known VSD.
I knew about the problem since the patient told me about it and it was mentioned in the chart. I started with Parasternal Long axis and looked for a flow by using Doppler. Once I found it, I used PW and CW to get the velocity. Then moved to Parasternal Short. Since the VSD was at 11 o’clock, it was an indication that it was a perimembranous VSD. Use color Doppler, lower the scale, take pictures and PW and CW too. Same in Apical 4, 5 and 3. Later I went to ask the MD if they needed contrast. Which I used later. Don’t have pictures here because the color Flow didn’t look good. But I had similar velocity. And always try a higher window at the parasternal. Instead of PEN, use GEN. The color Doppler will look better.